Describe the Particles Inside a Solid
Molecules slide past each other samples take the shape of the container. Particles in a solid are usually packed close together with a regular arrangement.

The Layers Of The Earth C Copyright 2006 M J Krech All Rights Reserved Outer Core Earth Convection Currents
Use a formula to justify your reasoning b which motion results in the.

. The particles are held together too strongly to allow much movement but the particles do vibrate. Particles behave very differently in the three stages of matter. Which of the following best describes particles of solid.
Solid are tightly packed usually in a regular pattern. Particles of Solids How will you describe the particles in liquid. Above this temperature the solid has become a liquid.
Use a formula to justify your reasoning. Solids contain particles that are tightly packed with very little space between particles. The amount of energy that the particles lose from these collisions is negligible.
Linear motion vibration and rotation identify. There are no attractive forces between particles. Linear motion vibration and rotation identifya which motion results in the particle having Kinetic energy.
In solids particles are very closely packed in an orderly arrangement in a lattice and only vibrate in fixed positions. In the liquid state particles can move - the movement temperature dependent tends to. Particles fill whatever container it is in.
The particles collide with each other and with the walls of any container in which they are held. Particles are tightly packed together. The forces of attraction between them are very strong.
Q1 Briefly describe the difference between the types of solids based on the arrangement of particles inside a solid. Particle Motion in a Solid. Particles are even more spread apart in.
The temperature at which this happens is called the melting point. In a solid the particles pack together tightly in a neat and ordered arrangement. Paul Ernest Z.
In the solid state particles are held closely together and vibrate slightly. Up to 24 cash back Draw three pictures of what the particles inside a solid liquid and gas may look like in the circles below. Describe the properties of a metallic solid.
Liquids and solids are often referred to as condensed phases because the particles are very close together. The particles in liquid are Figure 2. If the solid is heated the particles vibrate more and more until the force of attraction between them is overcome.
Particles in liquids are able to slide past each other or flow to take the shape of their container. The particles in solid are A Figure 1. This makes them very strong and difficult to break apart.
These particles are in constant random motion 3. Linear motion vibration and rotation identify a which motion results in the particle having Kinetic energy. The three different forms in which matter can exist are solid liquids and gas.
While they do vibrate slightly they do not move from place to place. On the other hand in liquids the particles are fairly packed with the minimal attraction between them. Q2 Among the three types of motion of particles.
Q1 Briefly describe the difference between the types of solids based on the arrangement of particles inside a solid. Moreover they are strongly attracted to each other. In liquids there are bigger spaces between particles and they move more freely and take the shape of their container.
Electric field inside the infinite plate is given by E 2ρrε0. Q2 Among the three types of motion of particles. They exist in a regular arrangement - there is no regular arrangement in the other states.
Solid Liquid Gas STATES OF MATTER. All matter is composed of tiny particles atoms molecules and ions 2. Solids can also hold their own shape The particles dont move around very much but simply vibrate.
Solid vibrate jiggle but generally do not move from place to place. Particles are arranged and move differently in each state of matter. HW-04 Q1 Briefly describe the difference between the types of solids based on the arrangement of particles inside a solid 02 Among the three types of motion of particles.
How will you describe the particles in solid. In solid the particles are closely packed together. They are fixed positions and they do vibrate.
All matter is made of up particles these particles behave in different ways whether they are solid liquid or gas. A solids volume and shape are fixed which means the particles are rigid and stay in place. Solids Particles in solids are held together very closely.
Gas vibrate and move freely at high speeds. Particles are tightly paked together is the best describes particles of solid. 310 describe the effects of changes in surface area of a solid concentration of a solution pressure of a gas temperature and the use of a catalyst on the rate of a reaction 311 explain the effects of changes in surface area of a solid concentration of a solution pressure of a gas and temperature on the rate of a reaction in terms of.
In a solid the particles can vibrate but they cannot move from one place to another. Use a formula to justify your reasoningb which motion results in the. A which motion results in the particle having Kinetic energy.
Liquid vibrate move about and slide past each other.

Representing Solids Liquids And Gases Using Particulate Models Video Khan Academy

Arrangement Of Particles In Phases Of Matter Comparison Expii

The Arrangement Of Particles In Solids Liquids And Gases Edukite Learning Youtube

Physical And Chemical Changes Science Freebie Matter Science Matter Worksheets Science Worksheets

6 2 Solids Liquids And Gases Particle Model Of Matter Siyavula
How Are Particles Arranged In The Three States Of Matter Quora

Arrangement Of Particles In Phases Of Matter Comparison Expii

Best Answer Draw A Diagram To Show The Arrangement Of Particles In A Solid A Liquid And A Gas What Brainly In
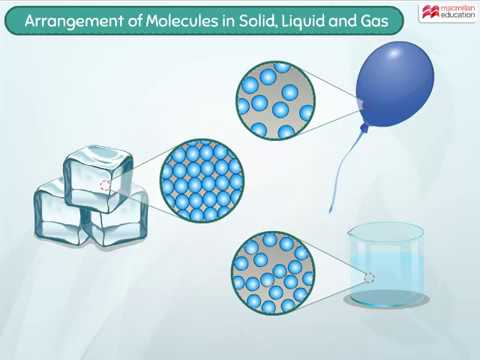
Arrangement Of Molecules In Solid Liquid And Gas Youtube

Solid Liquid Gas Phases Changes Venn Diagram Sorting Activity Public 2 Venn Diagram Activities Venn Diagram Matter Science

This Is A Concept Cartoon On Matter As Particles Particles Concept Bullet Journal
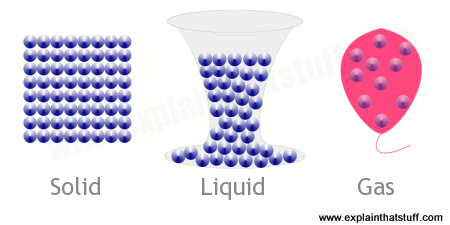
States Of Matter A Simple Introduction To Solids Liquids Gases

Arrangement Of Particles In Phases Of Matter Comparison Expii

Solid Liquid Gas Poems Homeschool Science Experiments Science Poems Matter Science

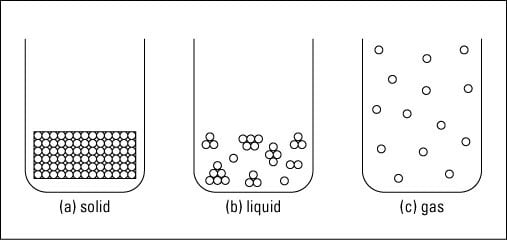
Comments
Post a Comment